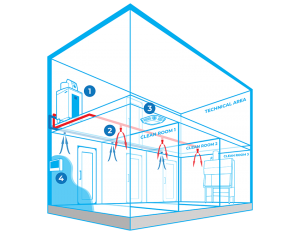
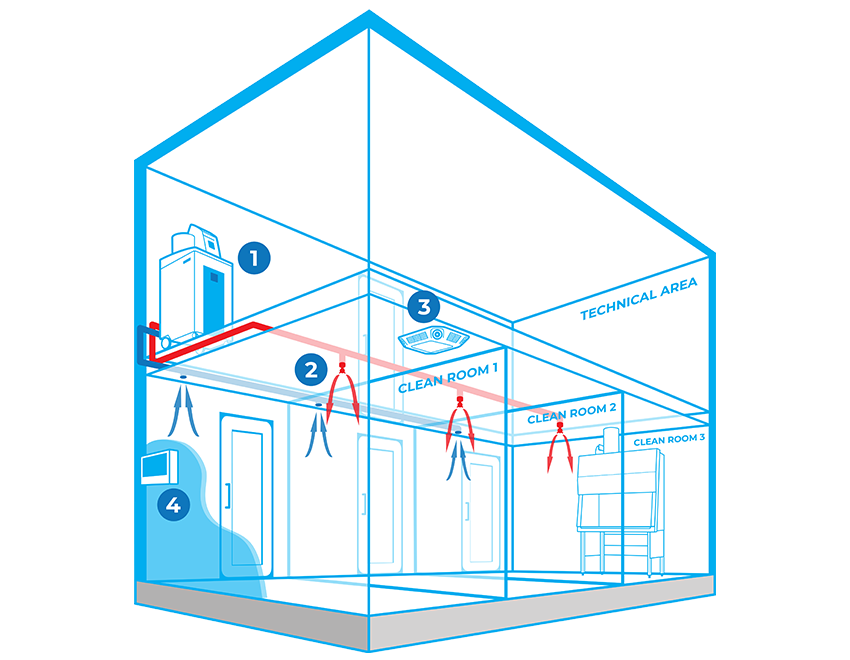
Bioquell Integrated Building Decontamination System
The Bioquell Integrated Building Decontamination System is a fully integrated solution designed for frequent or routine decontamination of the same area within a facility. This could be anything from a containment lab or multiple material airlocks, to a sterile manufacturing core or biomedical holding area.
Typically, Bioquell hydrogen peroxide vapor generator(s) are located centrally in a technical or unclassified space, and the vapor is distributed to the areas where it is required either through the building’s ventilation system or a dedicated network of pipes leading to nozzles on the ceiling of the target rooms. With an integrated system, no movement or storage of mobile equipment is required reducing manual setup times, increasing repeatability and removing human error.
Ideal for:
- GMP Biopharmaceutical manufacturing areas
- GMP Filling suites
- Biomedical facilities
- Biosafety/containment laboratories
WHY CHOOSE THE Bioquell Integrated Building Decontamination System
Efficient
No mobile equipment to store or set up in classified area meaning no trailing cables or sealing of doors is required, removing human error

Effective Distribution
Vapor can be distributed either using the building’s ventilation system or through a dedicated network of pipes

Automated
Can integrate with BMS and utilize actuated valves to control the delivery of vapor into each individual room, allowing a number of different decontamination cycles to be run at the touch of a button
Rapid
Quicker setup times, faster decontamination cycles and the ability to run the system overnight means less downtime

Efficacious
Validated and programmed cycles ensure repeatable 6-log sporicidal kill with Bioquell Hydrogen Peroxide Sterilant (EPA Registration Number: 72372-1-86703)

Safe
With the equipment located in an unclassified area, service and maintenance is easier and no personnel entry is required into a potentially contaminated target area
APPLICABLE SOLUTIONS
Bioquell Expertise with you from Concept to Completion
Following installation, every Integrated Building Decontamination System undergoes rigorous testing by our team of experienced validation engineers to ensure handshaking/communication to the building management system. Validation efforts ensue with the performance of an Installation and Operational Qualification (IQ/OQ) and customized 6-log sporicidal kill for cycles of any scale developed to the client’s specific needs. After testing and confirmed results, a fully functional decontamination system delivering repeatable results with high efficacy is available to use with the touch of a button.
Components

BIOQUELL INTEGRATED BUILDING DECONTAMINATION SYSTEM COMPONENTS




ACCESSORIES

Bioquell L-4
Use Bioquell’s versatile, multipurpose Hydrogen Peroxide Vapor generator to integrate into your facility in order to decontaminate areas on a consistent basis.

Use Bioquell’s versatile, multipurpose Hydrogen Peroxide Vapor generator to integrate into your facility in order to decontaminate areas on a consistent basis.

Bioquell Hydrogen Peroxide Sterilant (EPA Registration Number: 72372-1-86703) for use with the Bioquell L-4 delivers a 6-log sporicidal kill with traceability and auditing through RFID label technology
FAQs
What does a 6-log microbial reduction mean?
Log reduction (“log” is short for logarithm) is a numerical expression of the effectiveness of a decontamination procedure. A 1-log reduction is equivalent to a 10-fold or 90% microbial reduction; a 6-log reduction is a 99.9999% reduction.
How many spores are on a Bioquell Biological Indicator?
Each carrier disc found in a Bioquell Biological Indicator has over one million Geobacillus stearothermophilus.
What does the Tyvek® pouch do for the Biological Indicator? Is it needed?
Biological Indicators are pouched for many reasons, including ease of handling, ensuring microbes cannot be put onto the disc prior to use and aseptic handling after use. The crucial feature of the packaging is that it allows vapor to pass through, retaining the BI’s integrity.
What is a D-value?
A D-value is a measure of resistance. Bioquell’s D-value method shows the time it takes for 90% of the spores on the BI to be killed (also called a 1-log reduction). We aim for a D-value of one to two minutes.
Will I receive a certificate of analysis with Bioquell BIs?
The certificates for Bioquell BIs, including all independent verification, are all available on the Bioquell website. You can find them here.
What pack sizes are available for Bioquell Biological Indicators?
Our indicators come in boxes of 20 (one wallet of BIs) or 100 (five wallets of BIs); each re-sealable wallet contains 20 BIs.
Who makes the Biological Indicators?
We make all of our Biological Indicators in-house.
What pack sizes are available for Bioquell Chemical Indicators?
Our indicators come in boxes of 20 (one wallet of CIs) or 100 (five wallets of CIs); each re-sealable wallet contains 20 CIs.
How do the Bioquell Chemical Indicators work?
Each indicator has been formatted with a dye that will change color in designated areas on the card when exposed to Hydrogen Peroxide Vapor. The color changes provide a real-time indication of the success of your cycle.
Who makes the Chemical Indicators?
We make all of our Chemical Indicators in-house.
What concentration of hydrogen peroxide do you use?
Our systems and services operate using 35% hydrogen peroxide.
Why does Bioquell have RFiD-tagged peroxide?
Tagging the peroxide has several benefits: easy recording of volume used and volume remaining, traceability for GMP documentation purposes, quality assurance and legality of peroxide for cycle reproducibility and equipment longevity.
Where do I get the peroxide from?
High-quality and fully traceable peroxide is provided by Bioquell in safe-to-use bottles.
What gloves do you recommend for handling hydrogen peroxide?
Nitrile, PVC and neoprene single-use gloves should be used.
What is Bioquell RBDS?
Bioquell’s Rapid Bio-Decontamination Service is a fully inclusive and managed decontamination solution that uses Hydrogen Peroxide Vapor to eradicate microorganisms from the environment. It can be used for a multitude of reasons. Learn more here.
Will I have to close the whole building during this process?
Typically all adjacent areas can remain occupied with no disruption to your other working areas. We will also monitor the exterior of the area during decontamination as part of our safety procedures.
Is my space too large or difficult to decontaminate?
Whether you have operational and logistical concerns or the scope of the project is expansive, we will most likely be able to meet your decontamination needs thanks to the adaptability and scalability of Bioquell RBDS.
Can I leave my equipment in the area?
Yes – and we encourage you to do so. Equipment that has been removed will then have to be put back, adding extra work and risk of introducing a contaminant.
How long will it take for Bioquell to decontaminate my area for me?
Each situation is unique and we will work with you to provide an accurate assessment according to our knowledge from experience.
What type of documentation do I receive with this service?
RBDS is supported by documentation including full site surveys, safety information, risk assessment, method statement, detailed reporting confirming results and more. Visit the RBDS product page to see more.
Can you decontaminate equipment only with this service?
Absolutely. Please contact us to discuss further.
What will I need to do to make sure I can link this to my equipment?
We work with you to ensure everything you need is ready to decontaminate your equipment, from the necessary accessories to the connections required.
What is the maximum room size the Bioquell L-4 can decontaminate?
With the optional distribution head, the Bioquell L-4 could be used to decontaminate rooms up to 250 cubic meters. Contact us for more details.
When will I need a distribution head?
If you are looking to extend the capabilities of the system to decontaminate an open space, such as a room, then the distribution head would be required.
Can I connect this to my Building Management System?
Yes.
When will I need a Booster Fan?
If you are looking to decontaminate large filter bank enclosures with multiple filters, or single filter systems in a quicker time, then the Booster fan would be recommended.
The booster fan can also be used in small fixed room decontamination systems to reduce cycle times.
What will I need to do to make sure I can link this to my equipment?
The Bioquell IG-2 requires our engineers to work with you closely to fully integrate the system to your needs. Each situation varies, and we work closely with you from the start.
How do I control the system?
The Bioquell IG-2 offers a remote-control screen that can be placed at the most convenient location.
Will this offer 21-CFR Part 11 compliance?
Yes, we are able to offer an audit trail software package to support compliance with 21 CFR Part 11.
What size room can the Bioquell Z-2 decontaminate?
While variables such as the amount of surface area in the room can impact the capacity, the Bioquell Z-2 can handle rooms up to 250 cubic meters, with the potential for much larger spaces under Bioquell’s advise.
How do you start the unit?
There is a removable lectern with a built-in touch screen that you place outside the zone for safe and easy control.
Can I connect this to my Building Management System?
Yes.
What is the internal floor of the Bioquell Qube made from?
The floor of the Bioquell Qube is constructed from 316 stainless steel to ensure resistance to scratching on the working surface.
If my processes or needs evolve, is it possible to modify the Bioquell Qube accordingly?
The Bioquell Qube has been designed to allow for certain upgrades to be easily added after installation, such as adding a glove tester, particle counter, active air sampling device, racking or material transfer device. These options can be budget-friendly and can enhance your system’s capabilities. If you have specific future needs in mind, let’s discuss them today to ensure we design a solution that meets your current and future requirements effectively.
How can I bring tubing or cables into the Bioquell Qube?
We have an option for fitting a floor-mounted one-inch triclover port in each chamber. This allows you to bring in liquid lines or cables securely into the body of the Bioquell Qube.
What sets the Bioquell Qube apart from traditional stainless steel options?
The Bioquell Qube is constructed of tough and hard-wearing polypropylene, which is resistant to most materials the unit is ever likely to come into contact with. Because of its construction, the Bioquell Qube can be installed and validated within 16 weeks and, in most cases with minimal invasiveness to your operation or workflow.
Can you set alarms for the temperature inside the Bioquell Qube?
Yes.
If I wish to change pressure from positive to negative, do I need to change the filters or take any additional action to ensure it is safe to use?
The Bioquell Qube can be switched between pressure regimes simply with the touch screen; no additional changes are needed.
Are there different leak tests programmed for the Bioquell Qube?
Yes, there are leak tests programmed for both positive and negative pressure.
Will installation and validation of the Bioquell SeQure disrupt my workflow?
No. Depending on the volume of the area and the level of equipment contained therein, installation and retrofitting of the Bioquell SeQure can be completed and validated within a week. Our technicians can work during non-operating hours if possible and permitted by the client. Specific timeframes will be given for each project.
Can the Bioquell SeQure connect to my Building Management System?
Yes. Our expert technicians will perform a walkthrough of your cleanroom to ensure your systems are compatible with the Bioquell SeQure for integrated HVAC and automated operation. What’s more, Bioquell experts provide continued support for systems that are already in place, ensuring full BMS connectivity and solutions for the lifetime of your system.
How compact is the Bioquell SeQure?
Vaporizer: 800 x 580 x 160 mm (31.5 x 22.8 x 6.3 in)
Bottle Module: 520 x 550 x 140 mm (20.5 x 21.7 x 5.5 in)
Wired Control Unit: 260 x 350 x 95 mm (10.2 x 13.8 x 3.7 in)
Is an aeration unit featured in the system?
Yes. The Bioquell Port II features a powerful inbuilt catalyst for the rapid break down of hydrogen peroxide vapour.
How do you ensure all of the load receives 6-log bio-decontamination?
It is important that all surfaces of the load are exposed and occluded surfaces are minimised, Bioquell provide a number of standard and customised racking to assist with this.
Is the hydrogen peroxide vapour generator included with the system?
You will need to use a separate Bioquell L-4 generator alongside the Port II. This generator can also be used to decontaminate isolators and other equipment in your facility.
Contact Us
To learn more about how Bioquell can fit your solution, please contact us.
The Americas
Ecolab Inc
702 Electronic Dr. Suite 200
Horsham, PA 19044
+1 215 682 0225
bioquellusorders@ecolab.com